We accelerate healthcare and life science innovation with digital project management that ensures efficiency, compliance, and measurable results.
Our mission
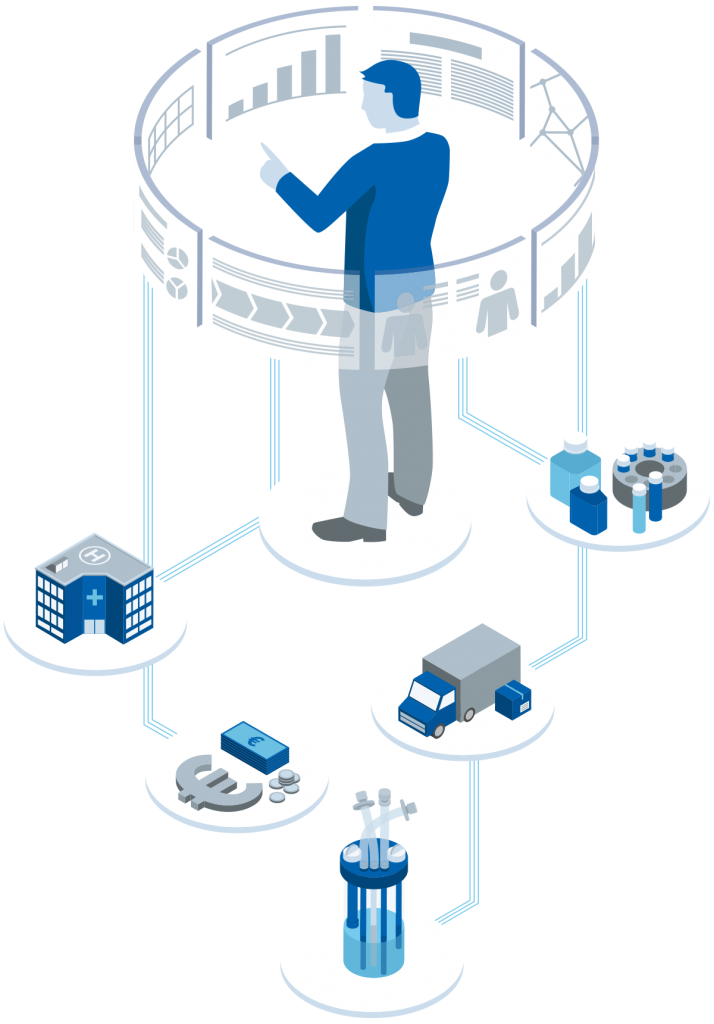
At cellerata, we enable and accelerate projects that improve patient safety, access to therapies, and organizational resilience. we bring our core competencies to the table: professional project management of therapeutics development and production, digitalization, and information security — always at the interface of biopharmaceutical products, therapeutic supply, and clinical applications. From bioprocessing and ATMP manufacturing to cybersecurity compliance, we help healthcare and life science organizations deliver innovation securely and efficiently.

Who we are
The company name reflects our approach: combining the precision of the cell, the smallest functional unit of life, with the speed needed to accelerate project performance. Considering client organizational background, we adjust speed, apply innovative PM tools to plan complex projects, implement them collaboratively, and deliver excellent results to senior management.
We are a flexible, dynamic, and agile consultancy that delivers proactive, efficient, and sustainable project execution. With five years of experience, we know that speed must always be adapted to the organization. By choosing the right tempo and the right tools, we help different types of organizations plan complex projects, implement them collaboratively, and deliver excellent results to management.
What sets us apart:
- Specialized expertise in development and biopharmaceutical manufacturing projects, focus on Advanced Therapies
- Strong focus on automation and digitalization of manufacturing and supply processes
- Extensive networking and partnerships across academia, pharma, and healthcare providers
- Certified in information (“cyber”) security as TÜV Nord NIS-2 experts
Accelerate your Cell Therapy project today
We are eager to discuss how we can successfully deliver your project at the intersection of bioprocessing, manufacturing, and medicine. As a first step, we offer you an initial project consultation — free of charge and without obligation. Together, we’ll identify opportunities, challenges, and next steps tailored to your situation.
Company profile
Founded 5 years ago, the Germany-based cellerata GmbH has successfully delivered a wide range of digitalization projects in healthcare and life sciences — from research-funded initiatives to pharmaceutical manufacturing and clinical collaborations.
I work closely with pm-praxis.de as expert in healthcare-IT infrastructure and applications. Together, we want to prepare organizations in the healthcare sector for digitalization along with security.
Our expertise spans the full innovation cycle:
- Evaluating Cell and Gene Therapy projects using value stream mapping, process optimization, production modeling, and resource simulations
- Driving digitalization in manufacturing with automated, closed solutions for greater efficiency and compliance
- Managing innovative projects that create value together with clients and partners in biotech and healthcare
- Ensuring cybersecurity protection for new digital assets, aligned with NIS-2 and BSI standards
We work closely with pm-praxis (experts in hospital IT), and bring certified expertise as TÜV Nord NIS-2 experts and registered IT service providers with the BSI.
Our approach
We believe that complex healthcare projects succeed when they are built on understanding, collaboration, and regulatory precision.
At cellerata, our approach combines:
- Listening first to fully understand customer demands
- Collaborative work styles that align teams and stakeholders
- Tailored solutions that take into account the relevant regulatory and legal requirements, applied with the knowledge and expertise to meet them in each project context (GxP, NIS-2, DSGVO, KHZG)
- Structured assessments to identify gaps, align on requirements, and agree on clear actions
This way, we ensure that every project is practical, compliant, and designed to deliver lasting impact.

Who we are
Dr. Thomas Appl – Founder & CEO
I founded cellerata to drive and deliver breakthrough Cell and Gene Therapy projects together with pioneering teams and organizations. For us, project management means guidance, leadership, and connectivity across multiple levels, always built on people working towards shared goals.
In highly regulated medicinal fields like Advanced Therapies is, projects often face technical and regulatory hurdles, driven by limited capacities and shifting governance structures, increasing economic pressure but at the same time, safety and quality first.
At cellerata, we address these challenges with a digitalized process-to-product modelling, simulations and solutions . These advanced analytics support further planning of products, processes and resources. In parallel we ensure with agile teamwork, transparent communication, real-time and bst practice information sharing.
While AI, machines, and humanoid assistance can support project execution, we believe that interdisciplinary teamwork is the true center of project excellence, and final decisions must always remain with humans. Through this approach, we create synergies along the value chain and secure project delivery as a true team effort.
Experts
Through our trusted network of senior experts, we provide support in areas such as business development, project management, technical operations, regulatory affairs, and quality management. We guarantee strict confidentiality while ensuring you benefit from deep, specialized expertise across the complex program lifecycle.
Clinics
At the end of the value chain, we work with clinical partners to ensure that therapies move
efficiently from production, supply to patient care. These collaborations add critical value to our services and help secure real-world impact for innovative treatments.