The Organisation
Over a decade, iFAKT GmbH established its proprietary Predictive ERP software solution to optimize productivity, line balancing and capacity utilization in the field of automotive and aerospace production (1). Cellerata GmbH partners with iFAKT GmbH to apply, model, simulate and analyze manufacturing projects of biopharmaceutical production of innovative therapies, in which time to patient is critical and conflicts of resources are evident. Specifically, cell and gene therapy projects entail additional project risks regarding biological variability of human tissue such as starting material, single batch productions and flexible capacities (2). Here, excellence in digital project management provides a helicopter view on the supply chain, process operations and resource allocation to the project team with control of budget, milestones and decision points to the project leadership.
In the present outline, we introduce iFAKT´s Integrated Manufacturing Solution Predictive ERP for a clinical project to potentially fight COVID-19 disease. We use convalescent plasma therapy as a recommended, safe, immediate and potentially effective intervention for COVID-19, which shows comparable project requirements with an allogeneic cell therapy project. Our case study provides insights how Predictive ERP can support drug developers, pharmaceutical manufacturers and clinical centers in planning a safe, time-critical and optimized clinical supply. Finally, we evaluate current limitations of critical capacities in the software and recommend optimization strategies based on our first process analysis.
The Project
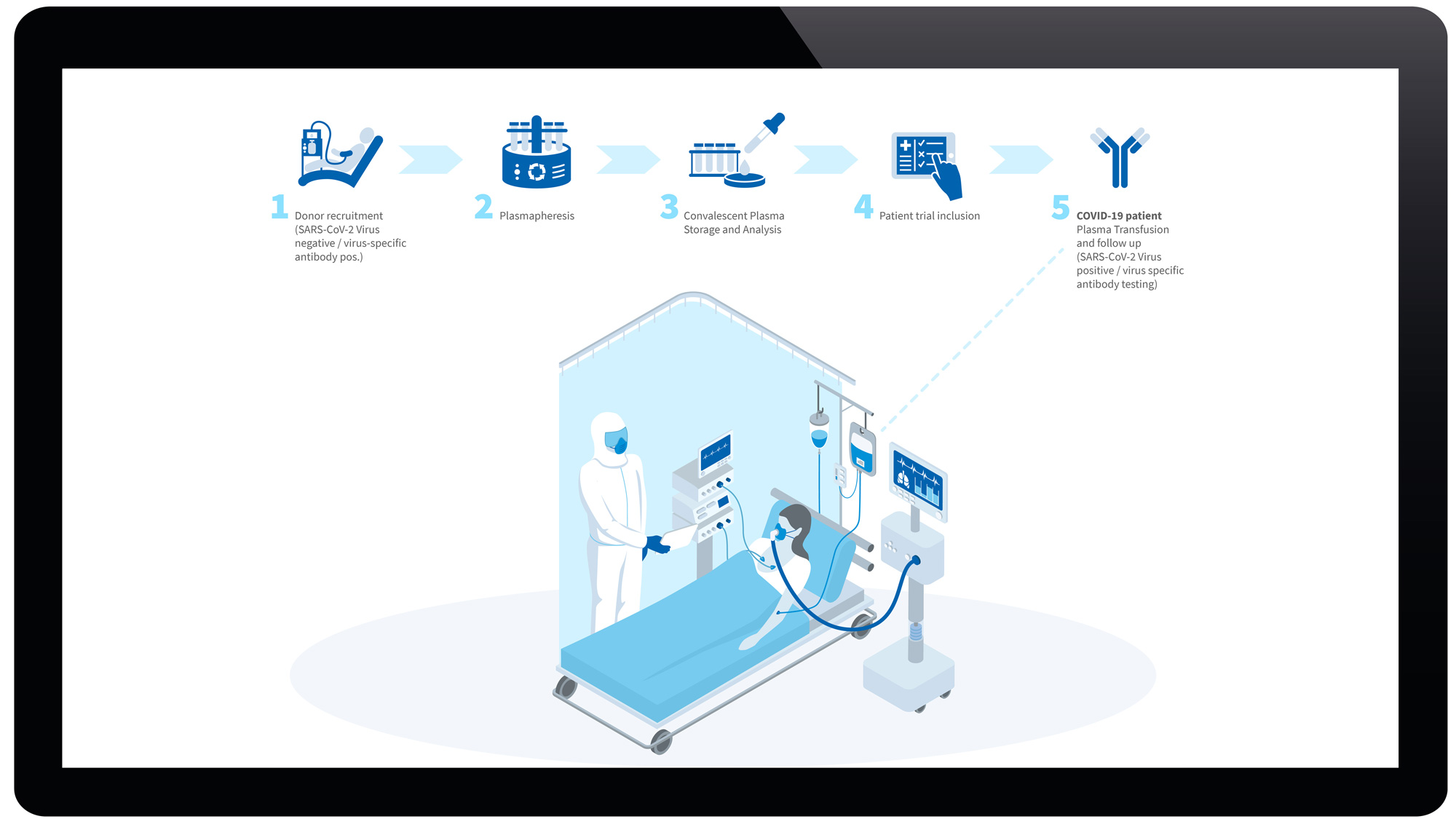
The SARS-CoV-2 virus is causative to COVID-19 and currently puts strong pressure on healthcare systems. High prevalence of severe COVID-19 cases with ventilation support in intensive care units (ICUs) already required a resource where demands exceeds capacity. Thus, patient turnover in ICUs limits rescue for as many patients as possible within their critical therapeutic window. While no SARS-CoV-2 specific pharmacological therapy is available on the market for COVID-19, convalescent plasma therapy has been shown to increase survival rates and reduces ICU stay in related virus infectious diseases. Immune, convalescent COVID-19 donors with a detectable virus-specific antibody serum titer against SARS-CoV-2 could be recruited for plasmapheresis. Convalescent plasma can be transfused in matched COVID-19 patients in ICUs as supportive therapy to rescue COVID-19 patients (3). We exactly want to model the process flow from donor plasmapheresis to patient treatment to simulate which bottlenecks impact patient treatment (Screen 1).
General project requirements
Several project requirements need to be managed by project management before production start.
First, legal, regulatory and organizational requirements need to be tackled by an interdisciplinary team of scientists and medical doctors from the transfusion center, plasma manufacturer and clinical center. This includes the submission of the clinical trial and the set-up of regulatory compliance in the Quality Management System.
Second, the number of severe COVID-19 cases, available and matched donors, access to non-infected personnel and limited ICU capacity need to be continuously evaluated with short reaction time. All relevant information needs to be collected in the Predictive ERP software to enable simulation of plasmapheresis, plasma manufacturing, and patient treatment.
Third, it is extremely important to orchestrate the supply chain from plasmapheresis and convalescent plasma supply at transfusion centers and patient treatment. This needs to be managed under restricted communication roles with limited direct contact of team players involved.
The software application Predictive ERP
We consider Predictive ERP as a digital platform for convalescent plasma production, logistics processes and patient treatment at the time of project evolution.
1. Plan products, processes and resources
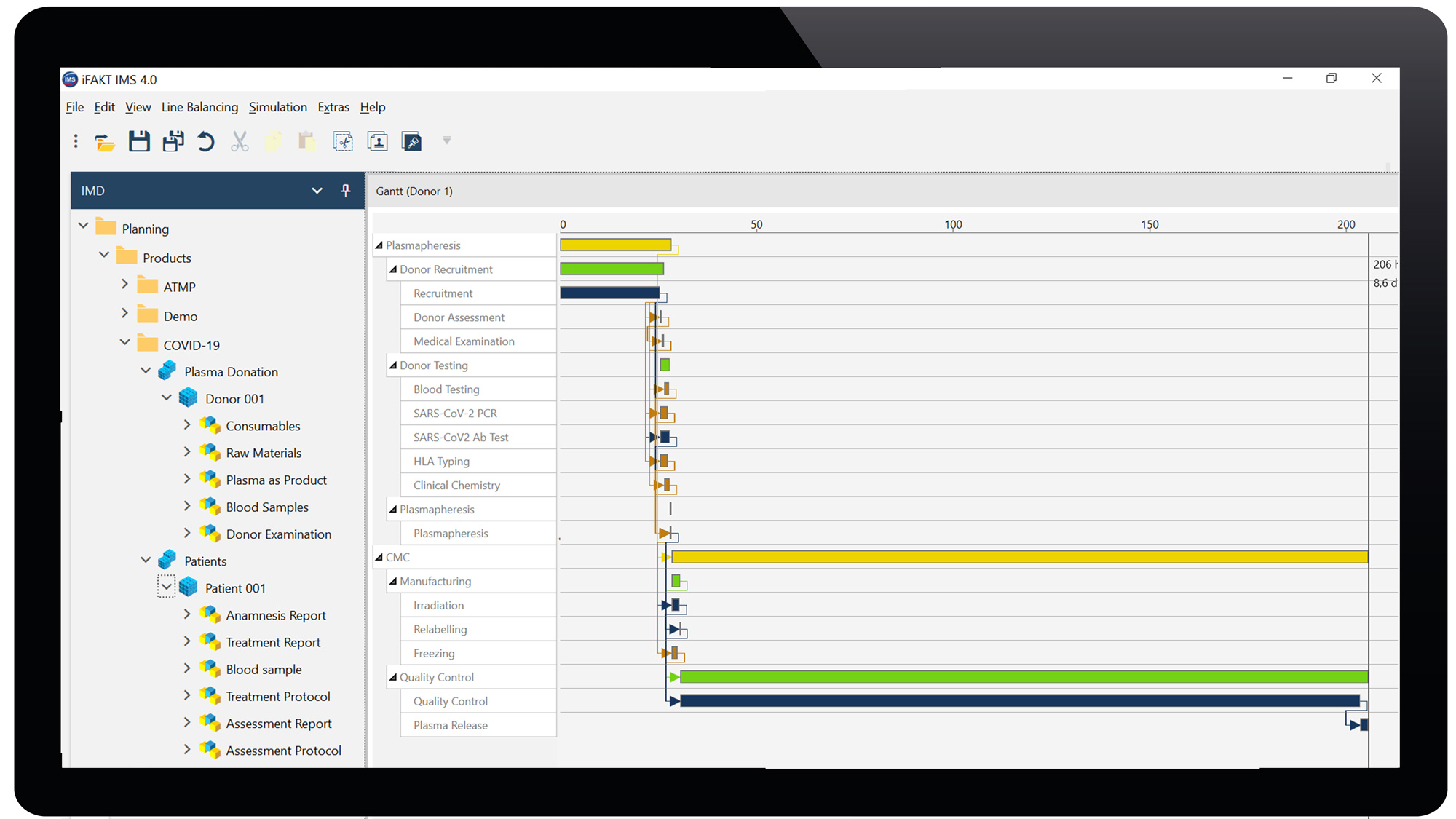
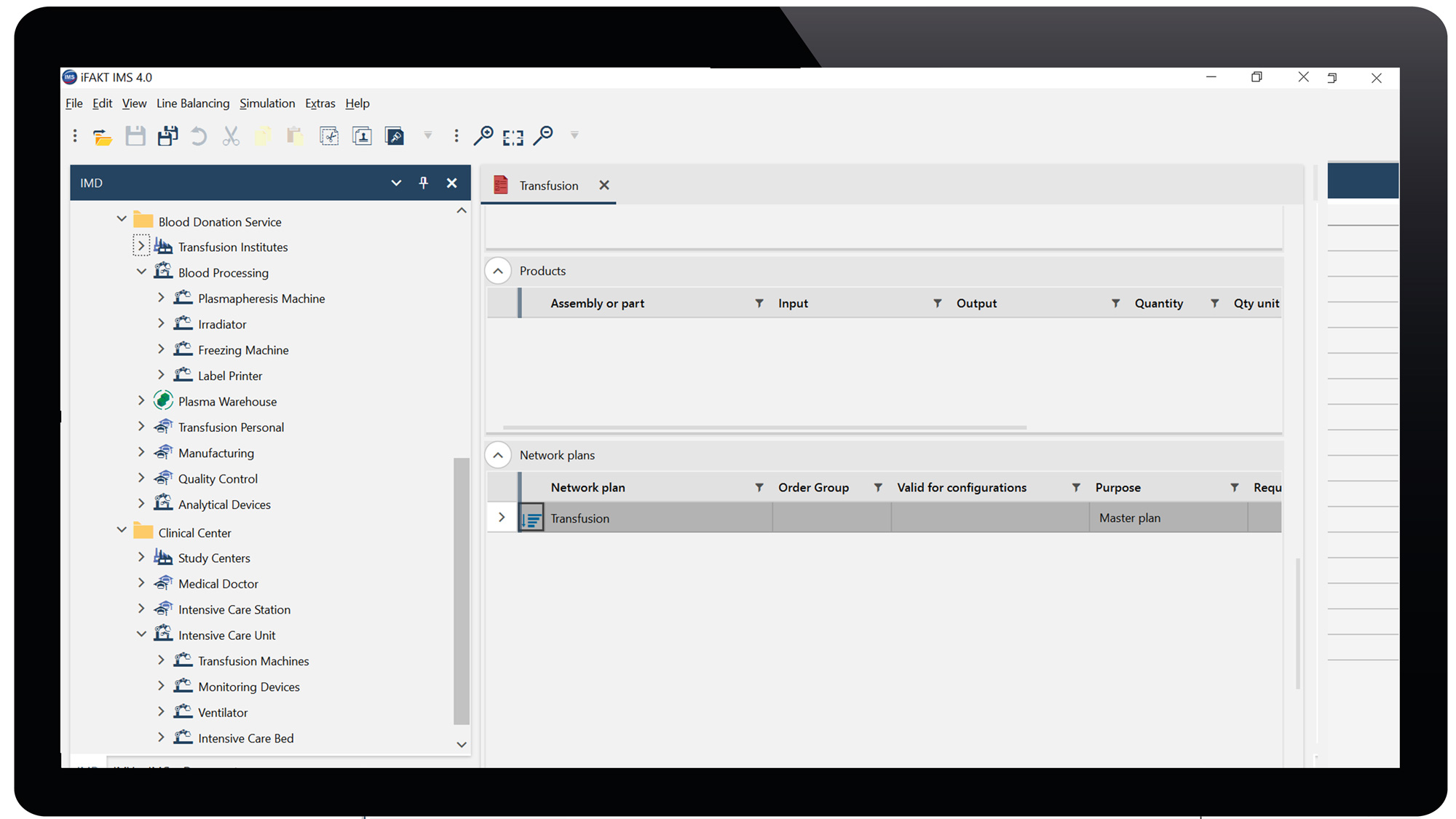
We feed the basic module IMDesigner (IMD) and start with a simple, but realistic data sets to methodically support various stages of planning including donor recruitment, plasmapheresis, manufacturing and patient selection, treatment and follow-up. We link processes with resources for simulation in a digital factory model across the interfaces from donor to patient. Pharmaceutical production, logistics processes, and clinical site management can be designed in a way that contains the essential and qualified resources and capacities. Furthermore, the interplay between raw materials, intermediate products and project activities with the supply chain is shown in comprehensive process charts with dependencies across the complete process. This results in network plans, determining throughput time and workload. The minimum throughput time, the critical path and the demand of resources are calculated with a static line balancing algorithm.
2. Simulation and scale up from an operational model to multicenter study
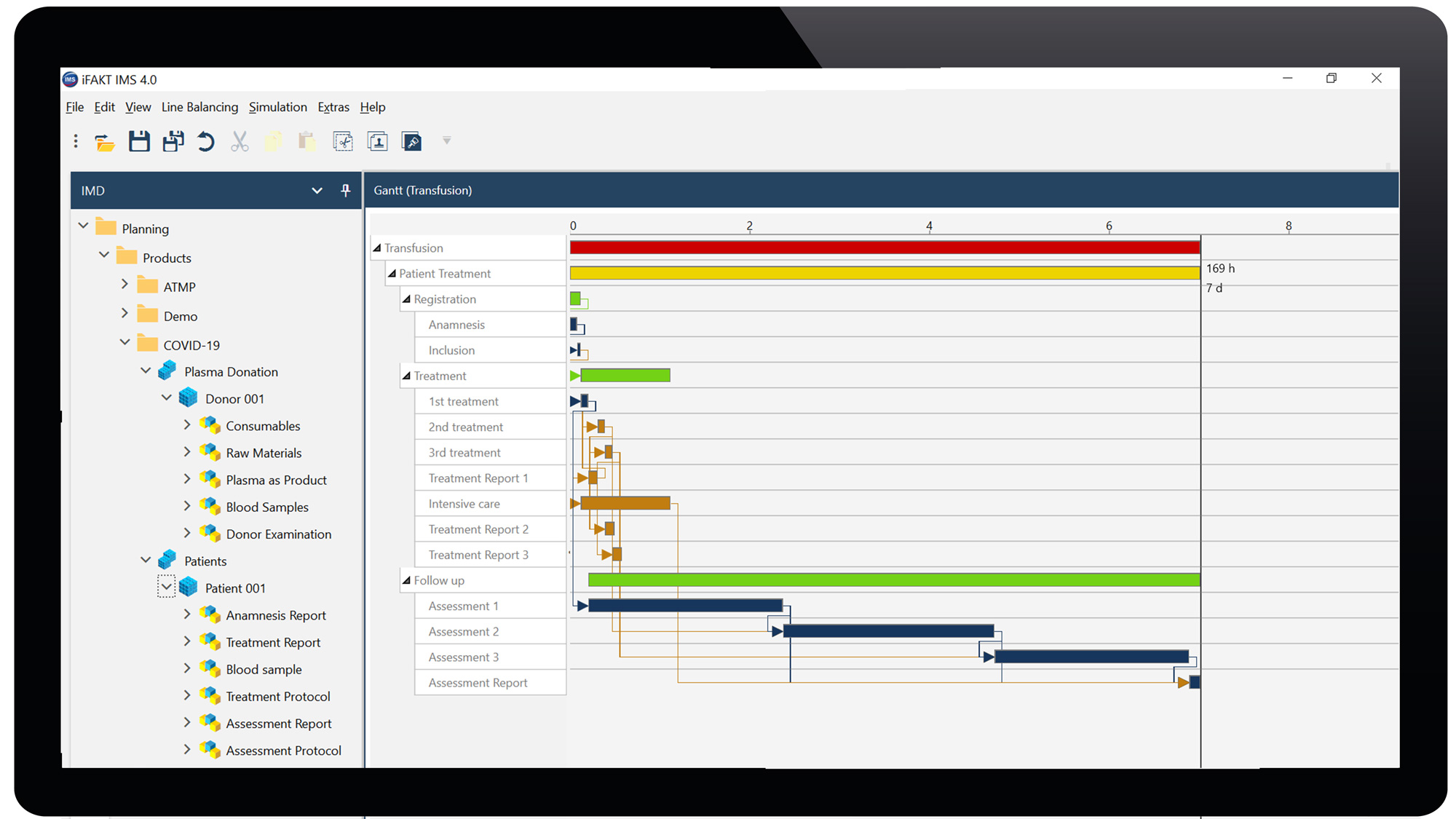
In the IMValidation (IMV) module, we simulate, visualize and validate various aspects of plasma supply and patient treatment with accessible information at the time of planning. We check, analyze and optimize plasma production and logistics processes before commencing a first plasma production at the transfusion unit. Due to complex interplay and dependencies, planning errors and capacity bottlenecks are modeled and thus identified in a one donor – one patient relationship. We compare different production scenarios based on bottlenecks in material flow, manufacturing resources, and personnel capacities to identify the best-case scenario covering the seamless value chain from needle to needle. The simulation model is the basis to scale up with multiple plasma productions from different transfusion centers and multiple ICUs treating patients in several clinical centers.
3. Perform Advanced Planning & Scheduling and operational Line Balancing
In the IMController (IMC) module, we perform Advanced Planning & Scheduling, operational line balancing and day-to-day planning for plasmapheresis and patient treatment. We optimize planning in detail by short-term simulations based on requests for convalescent plasma to have the possibility to react a in flexible manner to the availability of plasma. We optimize resource utilization by automated process allocation and establish in-time reporting, similar to a “shopfloor” at plasmapheresis center, pharmaceutical manufacturer and clinical site. Later on, this could provide real-time data and enables response to disruptions, setting up a building block system for responding to recurrent disruptions and faults in order to learn across clinical trial centers. Due to the high number of severe cases, we expect to include not only one but several centers for plasmapheresis and plasma transfusion, which increases complexity to another stage.
Screen 2: Projection of convalescent Plasma Therapy. From a voluntary donor tested positive for SARS-CoV-2 antibody, plasmapheresis is taken (2x). The Transfusion center processes, releases, provides 2 x 3 convalescent plasma units to the hospital. The intensive care unit transfuses 3 x 1 plasma units per each COVID-19 patient (n=2). Clinical outcome (i.e. survival, ventilation, cardiovascular, hematology, viral load, antibody titer) at 3 clinical assessment points. Gantt charts of plasmapheresis and patient treatment, resource allocation in small boxes (IMD module). Simulation of different scenarios with process optimization and scale up compiled in the IMC/IMV modules.
Conclusions
The Predictive ERP software modules allow presentation of a simplified, scaled 3D-layout of a product-to-clinic COVID-19 project across teams and sites. Using the software, we generate a blueprint of material flow and resource allocations from pharmaceutical production to the medical operations and we can analyze performance indices at different scenarios. We provide an identification of work overload under various conditions at the transfusion site, production site and in the clinical center. Information flow charts and graphs are systematically compiled in Predictive ERP with insights into process- and resource-related limitations. The most realistic scenario can be used for right-in-time resource planning, cost calculations and communication of planned timelines under the prerequisites and assumptions given.
From virtual to reality
Emerging R&D projects for the treatment of COVID-19 from product development, CMC manufacturing and clinical development need digital project management solutions for a systematic assessment of products, processes and resources. We now anticipate a COVID-19 project with an initiation phase of days using this project as a blueprint in which we adjust parameters to new conditions for an initial stress test. Virtual planning, production and treatment, as well as simulations, can be directly implemented in an actual operational mode. Furthermore, the Predictive ERP shop-floor module IMOperator (IMO) can be used as real-time feedback loop from operators at transfusion sites/ production sites and clinical centers to process data, work plans, and documentation. Project team members can provide their status information and report malfunctions. Data on availability of donors and plasma are systematically saved for precise scheduling and adjustments of production processes. This provides permanent transparency in the workflow and optimal capacity utilization. Microsoft Teams interactively connects multidisciplinary teams to share the project status in the cloud as a digital solution (4).
Our approach is essential for an immediate availability of innovative therapies and patient treatments, such as convalescent plasma for severe COVID-19 patients. The present show case underlines the need for a digital transformation of project management for Academia, Biotech and Pharma as well as their suppliers (5).
For further information please contact:
Dr. Thomas Appl, Managing Director cellerata GmbH t.appl@cellerata.com or
Lars Schubert, Managing Director iFAKT GmbH l.schubert@ifakt.de
References:
- www.ifakt.de/application/files/3715/6698/0726/white-paper-shaping-the-future-of-smart-manufacturing-with-predictive-erp-XS.pdf
- www.cellerata.com
- www.nature.com/articles/d41586-020-00895-8
- https://www.bearingpoint.com/files/BEDE15_0981_FC_EN_Digital_Transformation_final_web.pdf&download=0&itemId=186530
- www.ifakt.de