Among the ATMP family, Cell Therapy Medicinal Products (CTMPs), Gene Therapy Medicinal Products (GTMPs) and Tissue Engineered Products (TEPs) could have potential to shift the medical paradigm from symptomatic to curative treatment. Various autologous and allogeneic ATMPs such as Chimeric Antigen Representing T-cell (CAR-Ts) and Mesenchymal Stromal Cells (MSCs) are already available as marketed ATMPs. Most important, a large number of innovative ATMP candidates originated from different human starting material are in clinical development and take advantage of new viral vectors, genetic engineering using CRISPR/CAS, induced Pluripotent Stem Cell (iPSC) and human embryonic stem cell (ESC) lines.
While many of such approaches are promising for the field of Cell and Gene Therapy / Regenerative Medicine, academia and industry are facing several challenges:
1. Product complexity impacts production costs
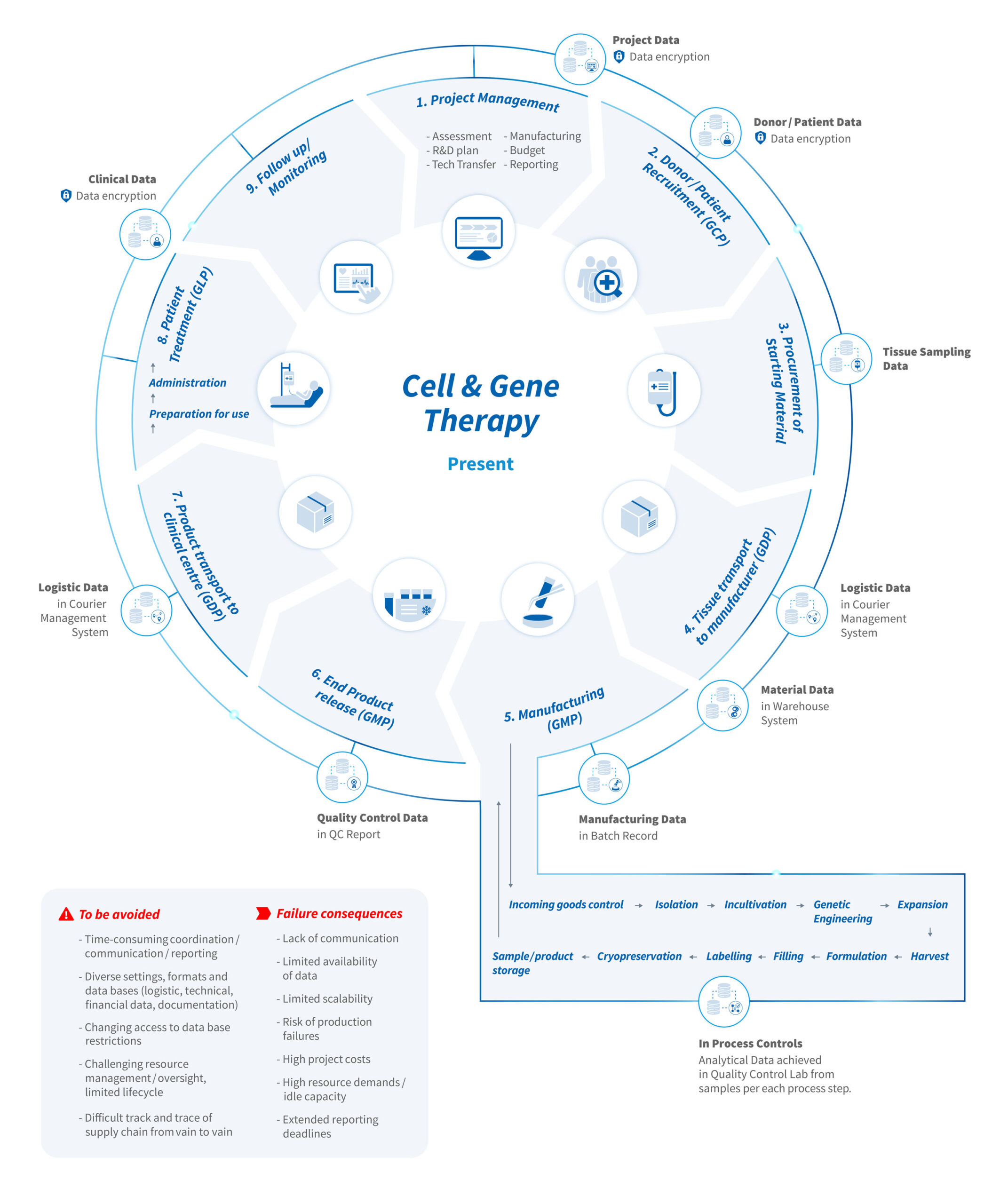
Most ATMPs are currently not produced off the shelf- they require product-specific development, coexistent with a regulatory and marketing strategy. ATMPs are refined across various biomanufacturing steps with a logistic chain from tissue procurement before manufacturing and back to the clinic for patient treatment. Especially, autologous, genetically engineered ATMPs, individually produced for a designated patient are among the most expensive therapies. Every ATMP undergoes not only a critical medical and quality review, but also a cost-value analysis from preclinical to market stage. At the same time, productions of ATMPs should be lean in their manufacturing process, economic in the costs of goods (CoGs) per each production, and de-risk in production failures.
2. The process determines product quality and quantity
Production of a cell-based therapy relies on availability of patients and in case of allogeneic therapies, availability of a voluntary, qualified, matched donor. Patient`s tissue itself entails biological variability for downstream processing to the final product. Furthermore, each raw material used in the manufacturing process needs to be qualified, tested and proven compliant to Good Manufacturing Practice (GMP). Operator-dependent, open process steps are also at risk for potential environmental contamination and “failed” batches. On the other hand, process optimization and scale up at late-stage clinical development require quality, technological and regulatory justification with a risk-based approach.
3. Seamless track and trace of materials, resources, and products
ATMP development programs that step across different clinical stages require dedicated and flexible resources guided by material and resource management. This includes detailed planning of material supply and warehousing, routine maintenance of equipment, and clean room reservations, especially if parallel productions collide for the same resource and ranking is required. Moreover, if products are manufactured at a Contract Development Manufacturing Organization (CDMO), the cell product needs interim stored and transported as stable and cryopreserved formulation with a safe cold chain logistics from the starting material to the patient treatment. Only precise and continuously planning of changing production scenarios under certain prerequisites and assumptions can risk mitigation a lack of therapy available for a patient urgently waiting for patient treatment.
4. ATMP production requires qualified resources in place
For each ATMP, a Pharmaceutical Manufacturer is authorized to produce under a manufacturing license obtained from local regulatory bodies. Each manufacturing process step and all associated analytical methods needs to be validated before clinical production. Translation and optimization of a manual manufacturing process from lab scale into a scalable, automated production platform requires experienced and qualified operators, scientist, engineers, data analysts, IT experts, Quality Managers, each providing their core expertise into a multifunctional project /production team. The interplay between human and technology resources allocated to a project team force a structural, organizational, communication architecture from material and technology suppliers, external labs, production to clinical sites and clinical trial sponsors (i.e. Clinical Centers, Biotech and Pharma companies).
The future of Cell and Gene Therapy
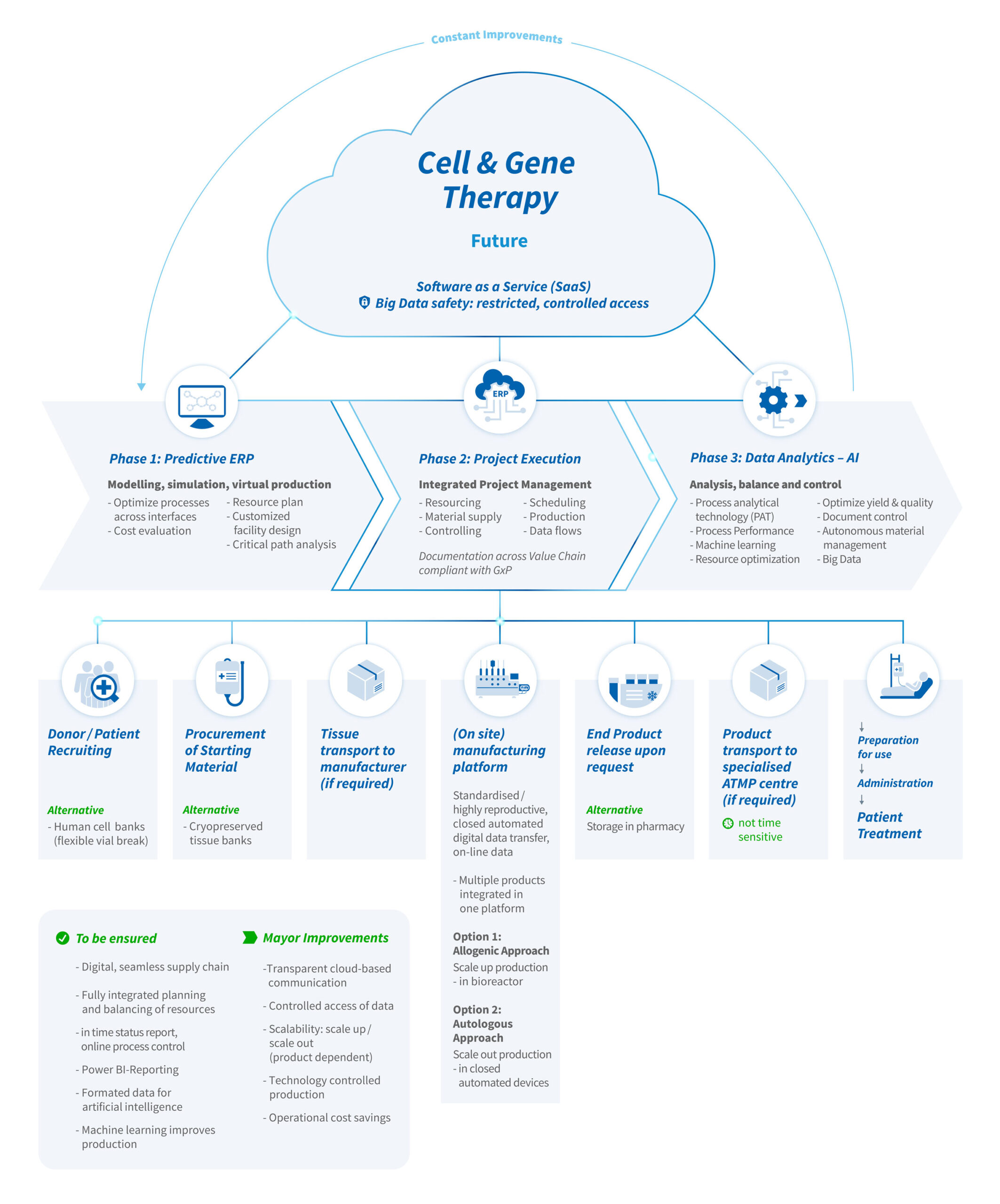
In the highly competitive environment of Pharma and Biotech, first in class ATMPs contribute to an unmet medical need, enable treatment of the first patient and the product license holder can successfully position the ATMP as a marketed product. In turn, this helps to apply the ATMP for other related indications or as successor products. Then established product platform will work based on a network of essential partners and outsourcing opportunities. In terms of production scenarios, more productions could be executed within a shorter time window (increase turnover while avoiding idle capacity). Under these conditions, the real value of ATMP production could only be achieved through a synergistic combination of scientific, technological, operational and digital solutions, in which the most competitive enterprise has full control over material supply, resourcing, technology, process, quality and budget. Any technological, processual, organizational changes need to be implemented stepwise to set the bar for a new industry standard in ATMP production.
Independent from science and technology, four steps will help to translate current manufacturing into automated production:
1. Standard sourcing of materials
At present, cells from a donor need to be matched for compatibility with the patient to avoid severe immune response such as cytokine storm. To avoid the dependence of availability of a suitable donor, advanced genetic engineering will be introduced to generate (immortalized or genetically controlled) human cell lines fully compatible and Human Leukocyte Antigens (HLA) matching may then be obsolete. Furthermore, cell vials from a cell line can be better characterized as starting material compared to primary cells, thawed under a standard operation protocol, and reproducible further processed. Finally, chemically defined cell culture media, compatible for thawing, expansion, cryopreservation will further help to obtain control over cell growth in suspended and adherent cultures on microspheres, biodegradable matrices and organoids.
2. Integrated production platform
If production is carried out in a specialized ATMP Center, ideally as an integrated production facility nearby a certified clinic for patient treatment, the risk of transportation from / to patient will be avoided. The production facility will not need to have highest biosafety level clean room capacities because productions can be executed in a platform using closed, automated, eventually later autonomous working units for scale out (autologous) or for scale up (allogeneic) productions. The platform approach enables assessment of data at critical process steps, comparison of data sets over productions, which can be subjected for Process Analytical Technology (PAT) to analyze data online and wireless for process control inside and outside the clean room. Massive data points generated from In Process Controls can directly feed into combinatory analytics, machine learning and artificial intelligence.
3. Predictive ERP – Predictive Enterprise Resource Planning
For detailed planning, a set of representative models for the diversity of ATMPs will be defined and implemented into a Predictive ERP software. In the software, the models are adjusted to each product for simulation of virtual productions ranging from material supply chains, production processes to product release and patient treatment in the clinic. Thereby, manufacturing and quality control can be reviewed in silico and in case of new products, a customized design of the process and eventually of the facility can be chosen based on most appropriate production scenarios. Manufacturing campaigns will optimize scheduling, critical path analysis will lead to precision in deliverable deadlines. Moreover, resource constraints or idle capacity will be avoided and indefinite cost estimations will be replaced by extrapolated cost calculations per each scenario. At the time of the first production, resourcing can be allocated based on automated production scheduling using verified and continuously improved algorithms, material supply will be controlled by autonomous ordering of raw materials and consumables. For all operations, an integrated project management will enable systematic communications transparently across interfaces and between humans and machines.
4. Analysis of production data
Integration of different sources and formats of data in one tank will open the possibility to compare key performance indices (KPIs) as critical performance and quality data from in process controls to the final product. The overall benefit of this development is to run every production safer and faster, being more effective through transparent and streamline communications in predefined settings. This should make ATMP production more reliable across batches and could significantly reduce the Costs of Goods per product, production, dose, and patient. The new standard of data driven productions should be compliant to global regulations and importantly, follow cyber security to protect personal data. Again, this transition of data streams into products will generate economically an even more attractive business field for the players involved in development, production, supply and application of the ATMP.
Simulations of production scenarios can only be done with a versatile, flexible tool, in which product, process, resource data are integrated across the vain-to-vain supply chain. The power of Predictive ERP is to model planning, allocate resources upon availability and qualification, to provide risk mitigation and to guarantee lean value streams by avoiding waste of resources. Most importantly, model simulations can be carried out virtually before major investments are taken and before the first production starts and thus, can uncover production planning failures.
For ATMP production, Predictive ERP could systemically delineate the following questions:
1. What is required to deliver the drug to the patient in time?
- What are the current lead times for provision of starting material and raw materials – and impact of absence of such materials on already reserved productions?
- What is the anticipated product yield per production given biological and process variability and what is the impact of failed productions?
- Which requirements are needed by when to deliver the ATMP across the planned clinical stages?
2. Can the manufacturer / product owner supply the expected number of batches with reasonable resources?
- Which resources limit ramp-up of production, especially if a process is optimized in parallel to ongoing productions?
- How can yet identified capacity constraints during scale-up be avoided by an appropriate outsourcing strategy?
- How should the ideal, product-specific facility be designed to assure a defined production load per month?
3. What will be the return of investment for the stakeholders involved in ATMP delivery?
- Which resources are required exactly to reflect different production scenarios?
- How can costs of goods and production costs be reduced over a sequence of productions?
- How do costs per unit (cost per dose) develop across scales?
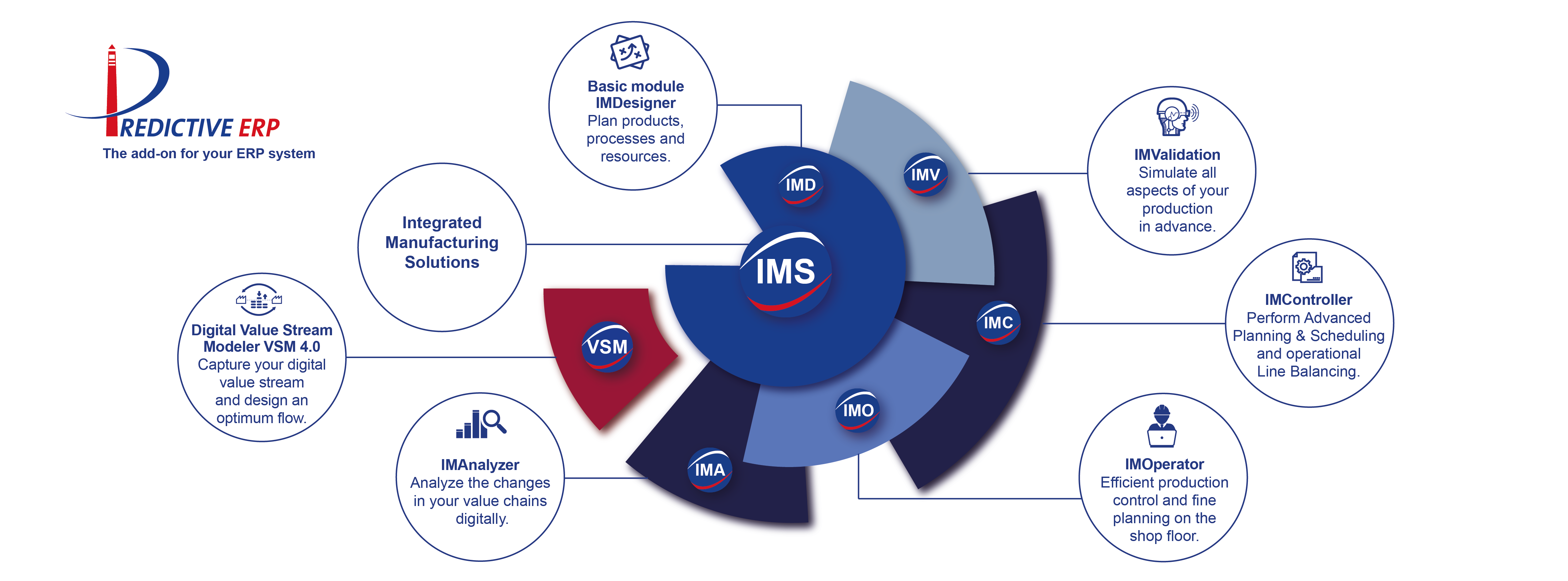
Modules of the Predictive ERP App – Suite
ATMP producing companies including CDMOs are confronted with challenges of product diversity, shorter delivery deadlines, production flexibility and consistent 100% quality per each ordered production request. We introduce, apply, and further develop highly flexible and safe online solutions for biopharmaceutical manufacturing, and explicitly for ATMP development and production. With the deployment of Predictive ERP, our clients (Pharma, Biotech, Academic Institutes, Clinical Centers, CDMOs, Technology providers) can better predict events of scenarios by today, as well as take planned measures and, in the process, adapt production and business processes in the future. PREDICTIVE analytics forecast models enable future-oriented decisions. By simulating scenarios in which even complex influences are taken into consideration, future developments can be forecasted and illustrated.
Integrated Manufacturing Solutions makes existing and anticipated digital production data usable profitably. Starting from a well-defined project plan in advance, accurate forecasts can be accomplished to execute the necessary actions automatically. The results of these real-time decisions are integrated by the system automatically and independently in intelligent, operative processes for continuous process optimization. By proactive identification and resolution of unexpected faults or disruptions, the business processes are optimized and as a result, designed significantly more efficiently, for example, for demand planning, maintenance, supervision, or pricing.
Integrated Manufacturing Designer (IMD) offers fast methodical support in the various stages of planning such as, for example, in the product analysis, resource planning and simulation, as well as manufacturing documentation. Processes can be design including all process steps from donor to patient (allogeneic) or from patient back to patient (autologous) as a seamless vain to vain chain. Set up a detailed and digital factory model for the production planning quickly and easily. Production plans can be prepared and designed as complex as desired: shorter sub-processes, products with more variants generated from a Process A to an optimized Process A` or an automated Process B. Both, manufacturing facilities with dedicated clean rooms for production of early stage clinical trials can be modelled and several facilities for multicenter studies engaged in clinical Phase III or market supply can be mapped.
Digital Value Stream Modeler VSM 4. – a tool developed specially for tablet applications, value streams are acquired quickly and easily in the production directly with the help of mobile touch-screen devices. Process-related information from operators (and later facility and equipment) can read off the automatically calculated key figures immediately. Processes and material flow as well as defects and weak points identified are documented and could be integrated into the Quality Management System. Target value streams are created directly on the touch-screen devices to monitor critical process steps and to separate value added from non-value-added process steps. The tool box enables direct comparison and simulation of different value streams and can be used to integrate the existing planning data with the actual production data.
Integrative Manufacturing Controller (IMC) module can perform Advanced Planning & Scheduling and operational Line Balancing in day-to-day planning. Detailed planning is optimized by short-term simulations based on current order information and thus, the production team can react in a flexible manner to fluctuations as well as enhance resources. This is especially important, if quantity/quality of the starting material is in the lower range of the specification the patient or the donor tissue as incoming starting material is delayed or postponed or by production shifts changes.
Integrative Manufacturing Operator (IMO) module can provide the production team with the process data, workplans and documentation via tablet. This is especially important if the production schedules require continuous and flexible availability of clean rooms, manufacturing and analytical equipment. Employees can provide status information and report malfunctions directly. The organization itself can increase the performance of productions by precise scheduling of the processes involved, permanent transparency, and optimal capacity utilization in the clean room and in the Quality Control laboratories.
Integrated Manufacturing Validator (IMV) provides dynamic simulation to analyze and improve production processes even prior to commencing production. You can check operative strategies for efficiency and compare various planning alternatives. The virtually safeguarded results permit well-founded and economically meaningful investment decisions to be made. Achieve transparency, increase productivity, and lowering of costs impacts a predictive and comprehensive view of planned and ongoing processes. With the help of material flow and resource models combined with statistically obtained demand and throughput parameters, the organization has a tool to predict both recurrent and basic changes and implement anticipatory measures.
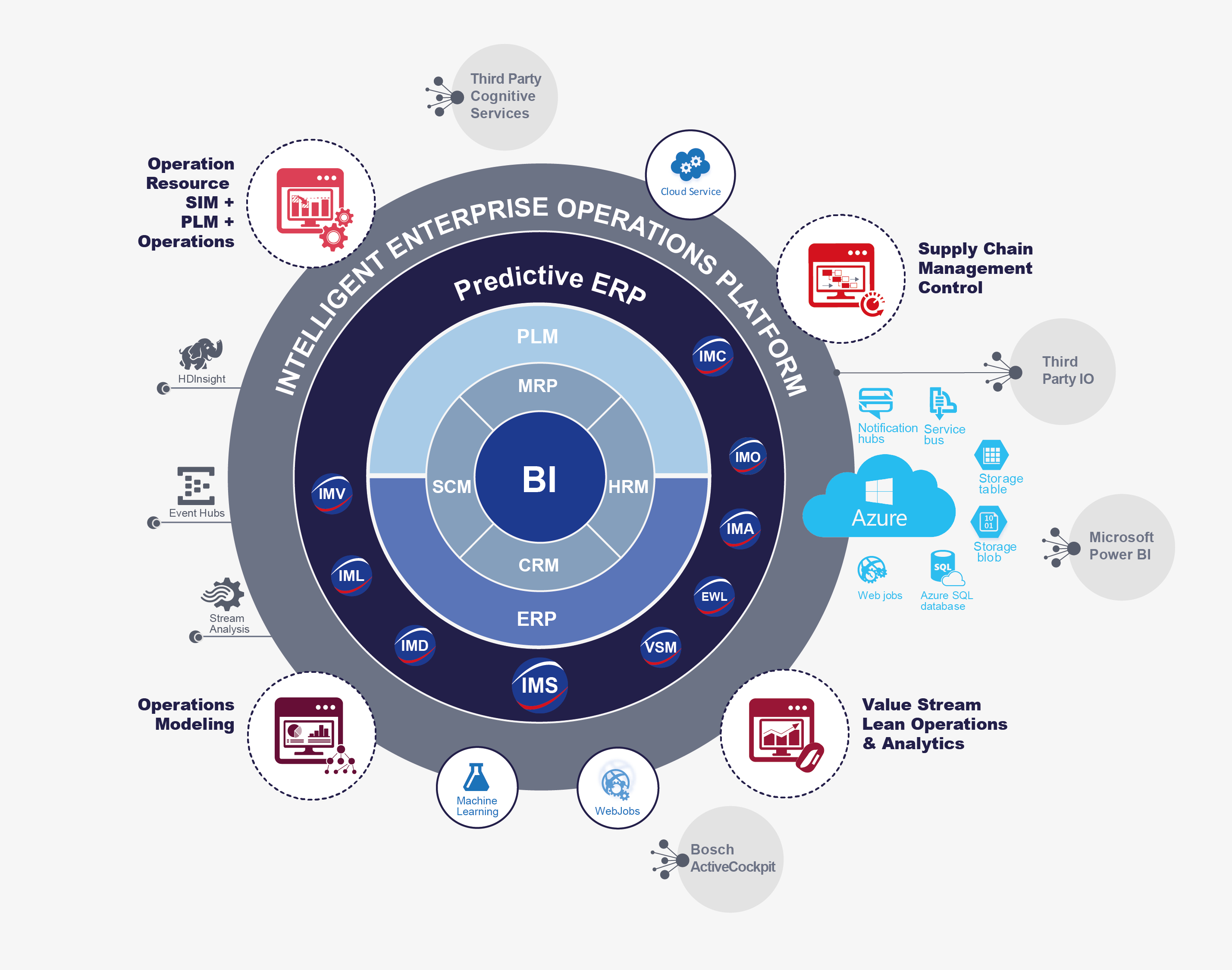
The software platform can be used as Software as a Service (SaaS) and web-based. This avoids server related costs at the user, workload and time for installation, introduction and servicing compared to server solutions. Depending on the size of program, number of administrators and users, the software licenses are provided in line with your needs with great flexibility. Multiple project partners can access the data online and collaborate at the same time. Data is stored on national servers in line with the most up-to-date data security standards and can be accessed any time and from anywhere.
The Future of ATMP production: Cell therapy 4.0
The future of the biopharmaceutical manufacturing is on the way of getting smarter: sensors are integrated into devices and equipment, wireless technologies and intelligent algorithms are used to process data. ATMPs will also profit from process standardization combined with process analytical technology (PAT) as key enabler to improve production processes and generate big data for use of Artificial Intelligence (AI) algorithms. This systemic approach has been shown to be successful in standardized and high- throughput branches such as automotive, aerospace, medical technology. ATMP production is on the way to fit industrial standards as well. Automation will be prominent for scale out autologous products and scale up allogeneic products. This development will corroborate with operational excellence, improved production efficiency, costs reduction and attractive price mark up of ATMPs.
Predictive ERP intelligently transforms operations management at all the levels of the company and across organizations working under the context of the Industry 4.0 (I4.0). Furthermore, its objective is to fill the previously mentioned gaps to fully benefit from advanced technologies data and I4.0. There were no constructive relationships between these backbone systems. Nonetheless, trends in the I4.0 paradigm propose to take advantage of data to manage the company’s operations as an unique system. Such unique system would be brain and heart of the company’s operations to have in-time delivery, product quality at the best possible cost structure.
In the future, biopharmaceutical manufacturing will also profit from I4.0 on the three major information layers: The Smart Factory, the Digital Thread, and the Value Chain Management. These layers themselves have sub layers enabling their functioning:
- The Smart Factory optimizes the flow of products through production processes and orchestrates the allocation of resources. This layer encompasses another five sub layers: Business Intelligence, connected Enterprise system, Operations Management (smart Apps, controllers), OT-IT Bridges and Smart Machines (sensors, tooling, workforce).
- The Digital Thread: It encompasses the creation of work instructions for the automated production and the verification of the processes: cell cultivation, genetic engineering, cell expansion, harvest. There are again three sub layers: Specifications management, Operations Management, and Service Management.
- The Value Chain Management: It delivers real-time data from production processes to other business management functions and merges activities into the supply chain. This makes sure that materials, intermediate products are further processed to the final product at the right place, time and resources (skill sets). It encompasses the five sub layers: Customer Management, Compliance Management, Operations Management, Resource Management and Supplier Management.
Predictive ERP can be the driver to assist biopharmaceutical production of ATMPs from development and production to the clinical application. To our clients, we provide services in the field of ATMP production using our Predictive ERP App-Suite:
- Digital lean management including value stream analysis, mapping, and design
- Evaluation of production start using simulation of various scenarios
- Sustainable efficiency increases in production due to optimized resource utilization
- Supply chain optimization through integrated planning of the value creation networks
- Increasing the production rate by analyzing and avoiding bottlenecks in the material flow
- Patient-centric, dynamic resource planning of seamless end-to-end business processes
- Line balancing and production planning through intelligent performance optimization algorithms
- Integrated shop-floor feedback taking operational production improvement into account
For further information please contact:
Dr. Thomas Appl, Managing Director cellerata GmbH t.appl@cellerata.com or
Lars Schubert, Managing Director iFAKT GmbH l.schubert@ifakt.de