We help biotech, pharma, and academic organizations deliver data, services, processes, products, and therapies faster and more reliably. With expert-guided, AI-supported project management, we streamline processes and information flows to create lean, efficient, and compliant project solutions — and connect technology suppliers with therapeutic experts in the clinic to ensure innovation reaches patients effectively.
Advanced Therapy Medicinal Product (ATMP) projects are complex, highly regulated, resource-intensive – and thus require high-end cost justification. By combining regulatory know-how, lean project execution, and a collaborative approach across the value chain, we help you move from early development to market-ready therapies.
Our focus spans the full journey:
From early therapeutics development to clinical implementation, with the final goal to reach market approval for indications, in which limited or no alternative treatment is available. We place particular emphasis on the implementation of these therapies in specialized clinical centers in Germany, ensuring that innovation reaches patients efficiently and safely.

Accelerated access of therapies for life-threatening diseases
Advanced Therapies are current and future drivers to address urgent, unmet medical needs. Symptomatic-based treatment as standard of care often falls short in treating life-threatening diseases. Cell and Gene Therapies have the potential to transform patient outcomes, but projects in this field are highly complex.
We support you in overcoming these challenges by:
- Bridging the gap between research, manufacturing, and clinical supply
- Ensuring compliance through in-depth legal, regulatory, quality, and security checks
- Conducting process assessments and optimizing execution with lean principles
Our mission in a nutshell:
Make innovative therapies more accessible, efficient, and sustainable. We address this in projects together with partners to optimize, automate, scale, and digitalize the value chain from production to clinical supply and application.
How cellerata operates
Project assessment and consultancy
Partner with cellerata and benefit from our expert project consultancy for your Cell and Gene Therapy project.
Our services cover:
- Product and process development — guidance from research through scale-up/scale out
- Technology transfer — smooth transition from lab to GMP manufacturing
- GMP production — ensuring quality and compliance at every stage
- Regulatory compliance — Prepare for readiness for audits, inspections, and filings
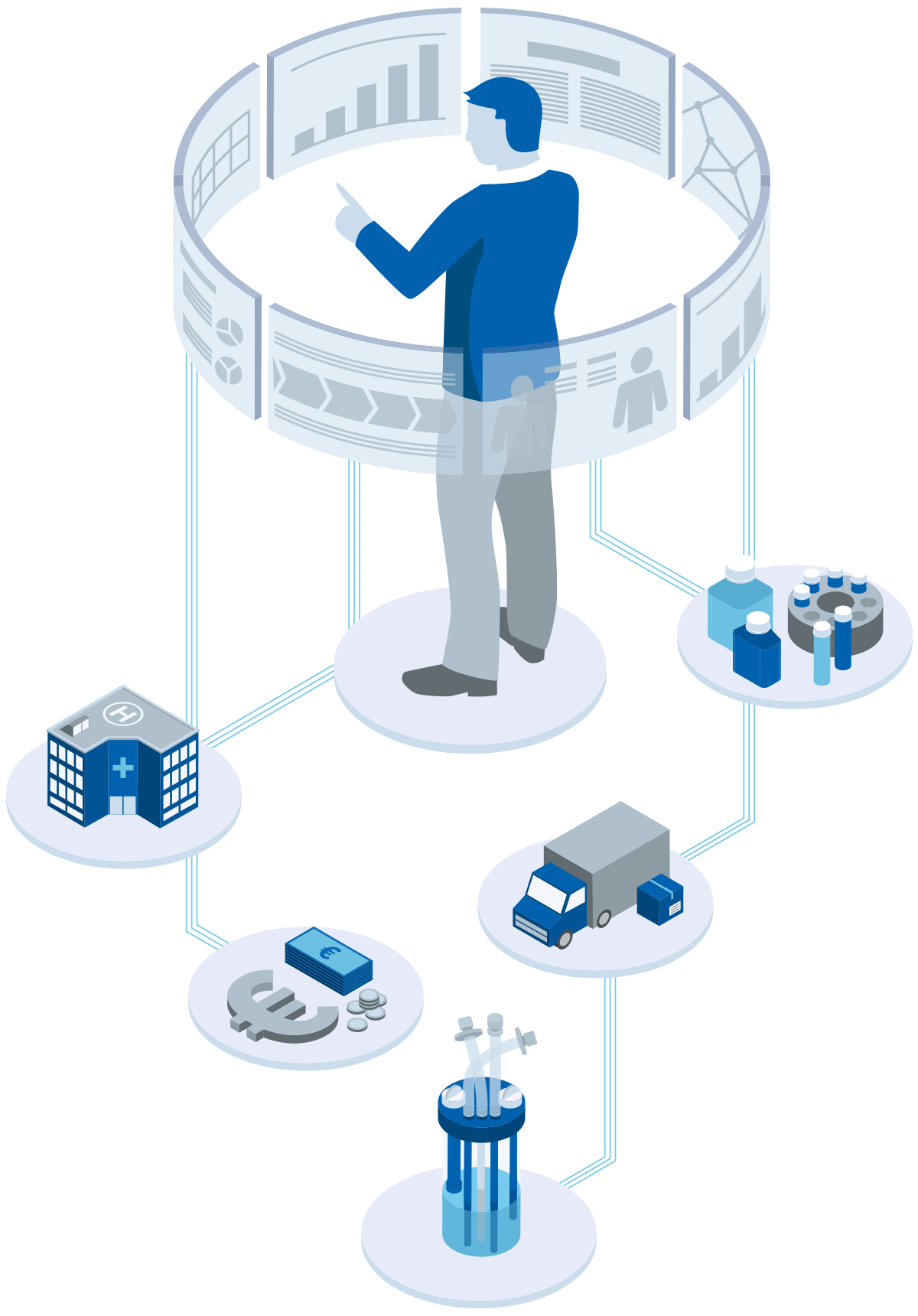
Operational project management
At cellerata, we turn plans into action. Our focus is on the operational execution of projects, ensuring that concepts become measurable results.
We provide:
- Project planning and transparent tracking to create a clear path forward
- Risk management and mitigation strategies for secure project delivery
- Team up with clients, internal teams, experts, and stakeholders to ensure projects are successful
- Project coordination for goal-oriented execution across all phases
- Condensed knowledge and know-how, documented as products, processes, and regulatory-ready documentation
Accelerate your Cell Therapy project today
We are eager to discuss how we can successfully support your project at the intersection of bioprocessing, manufacturing, and therapeutic application. As a first step, we offer you an initial project consultation — free of charge and without obligation. Together, we’ll identify challenges and opportunities tailored to project stage.
Why work with us?
We help organizations move faster and leaner in areas where CDMOs and large internal teams are often costly and slow. By combining regulatory requirements with practical execution, we deliver results that accelerate development and secure compliance.
Faster project execution
Support for developers with lean processes that run more efficiently in-house or through streamlined transfer to CDMOs
Efficient tech transfer
Enabling smooth transitions from developers to manufacturers, including US-to-Germany or US-to-Europe collaborations
Cost- and time-efficient services
Delivering expertise and execution without the overhead of high-cost CDMO staff
National compliance focus
Ensuring information security projects align with German regulations, just as pharmaceutical manufacturing must protect patient safety
Trusted partner in Germany
With most clients and commissioning organizations based in Germany, we combine local regulatory insight with international project experience